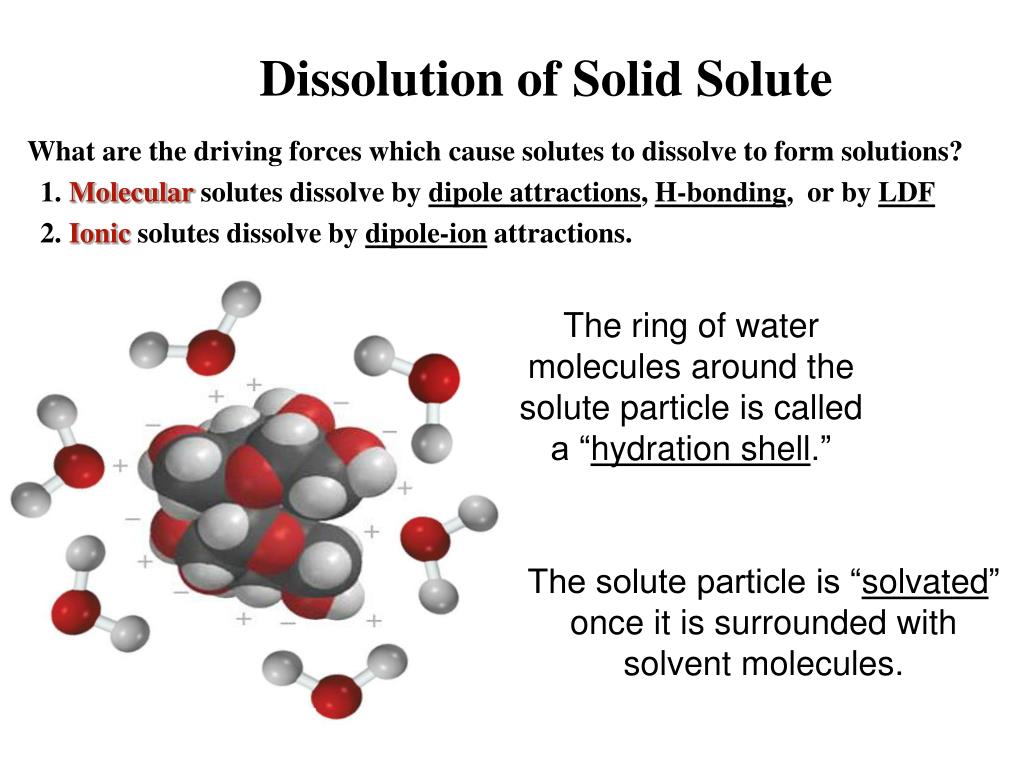
Do Solvents Dissolve Solutes . The substance that dissolves is called. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solvation is the process in which molecules of a solvent attract the particles of a solute. A solution is a homogeneous mixture consisting of a solute dissolved into a. The solute is the substance that is being dissolved, while the. A solution forms when one substance is dissolved by another. The solute is the substance that is being dissolved, while the. Solvents that are nonpolar will dissolve nonpolar solutes. Solvents that are very polar will dissolve solutes that are very polar or even ionic. When one substance dissolves into another, a solution is formed. A solution is a homogenous mixture consisting of a solute dissolved into a solvent.
from www.slideserve.com
Solvents that are very polar will dissolve solutes that are very polar or even ionic. A solution forms when one substance is dissolved by another. When one substance dissolves into another, a solution is formed. The solute is the substance that is being dissolved, while the. Solvation is the process in which molecules of a solvent attract the particles of a solute. Solvents that are nonpolar will dissolve nonpolar solutes. A solution is a homogeneous mixture consisting of a solute dissolved into a. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. The substance that dissolves is called. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution.
PPT Chemistry B Intro to Mixtures PowerPoint Presentation, free
Do Solvents Dissolve Solutes The substance that dissolves is called. A solution is a homogeneous mixture consisting of a solute dissolved into a. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The substance that dissolves is called. Solvation is the process in which molecules of a solvent attract the particles of a solute. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. A solution forms when one substance is dissolved by another. When one substance dissolves into another, a solution is formed. Solvents that are nonpolar will dissolve nonpolar solutes. The solute is the substance that is being dissolved, while the. Solvents that are very polar will dissolve solutes that are very polar or even ionic. The solute is the substance that is being dissolved, while the.
From www.expii.com
Soluble vs. Insoluble — Comparison & Examples Expii Do Solvents Dissolve Solutes The substance that dissolves is called. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. When one substance dissolves into another, a solution is formed. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. Solvents that are very polar will dissolve solutes that are very. Do Solvents Dissolve Solutes.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Do Solvents Dissolve Solutes When one substance dissolves into another, a solution is formed. A solution is a homogeneous mixture consisting of a solute dissolved into a. Solvents that are very polar will dissolve solutes that are very polar or even ionic. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. Solute the solid (or occasionally a gas) which. Do Solvents Dissolve Solutes.
From www.biomart.cn
Solubility Factors When Choosing a Solvent 企业动态 丁香通 Do Solvents Dissolve Solutes Solvents that are very polar will dissolve solutes that are very polar or even ionic. The solute is the substance that is being dissolved, while the. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. When one substance dissolves into another,. Do Solvents Dissolve Solutes.
From thechemistrynotes.com
Solvent Definition, Types, Incredible Uses, Examples Do Solvents Dissolve Solutes Solvents that are nonpolar will dissolve nonpolar solutes. The substance that dissolves is called. A solution is a homogeneous mixture consisting of a solute dissolved into a. Solvents that are very polar will dissolve solutes that are very polar or even ionic. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. The solute is the. Do Solvents Dissolve Solutes.
From energyeducation.ca
Solute Energy Education Do Solvents Dissolve Solutes Solvents that are very polar will dissolve solutes that are very polar or even ionic. Solvents that are nonpolar will dissolve nonpolar solutes. The solute is the substance that is being dissolved, while the. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Solute the solid (or occasionally a gas) which dissolves into a solvent. Do Solvents Dissolve Solutes.
From slideplayer.com
Solutions and Solubility ppt download Do Solvents Dissolve Solutes Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. A solution forms when one substance is dissolved by another. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. When one substance. Do Solvents Dissolve Solutes.
From pediaa.com
Difference Between Solvent and Solute Definition, Properties, Examples Do Solvents Dissolve Solutes The solute is the substance that is being dissolved, while the. The solute is the substance that is being dissolved, while the. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solvents that are nonpolar will dissolve nonpolar solutes. Solvents that are very polar will dissolve solutes that are very. Do Solvents Dissolve Solutes.
From www.youtube.com
Difference between solute and solvent YouTube Do Solvents Dissolve Solutes Solvents that are very polar will dissolve solutes that are very polar or even ionic. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The substance that dissolves is called. When one substance dissolves into another, a solution is formed. A solution is a homogeneous mixture consisting of a solute dissolved into a. A solution. Do Solvents Dissolve Solutes.
From www.dreamstime.com
Solute, Solvent and Solution Isolated with Red Solute Stock Do Solvents Dissolve Solutes Solvents that are very polar will dissolve solutes that are very polar or even ionic. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Solvation is the process in which molecules of a solvent attract the particles of a solute. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order. Do Solvents Dissolve Solutes.
From socratic.org
What is the relationship among solutions, solutes, and solvents? Socratic Do Solvents Dissolve Solutes A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. The solute is the substance that is being dissolved, while the. The solute is the substance that is being dissolved, while the. Solvents that are very polar will dissolve solutes that are very polar or even ionic. Solvation is the process in which molecules of a. Do Solvents Dissolve Solutes.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3746599 Do Solvents Dissolve Solutes A solution forms when one substance is dissolved by another. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. A solution is a homogeneous mixture consisting of a solute dissolved into a. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solvation is the process. Do Solvents Dissolve Solutes.
From slideplayer.com
Mixtures, Elements and Compounds ppt download Do Solvents Dissolve Solutes A solution is a homogenous mixture consisting of a solute dissolved into a solvent. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solvation is the process in which molecules of a solvent attract the particles of a solute. The solute is the substance that is being dissolved, while the.. Do Solvents Dissolve Solutes.
From www.vrogue.co
Ppt Solvent Solute Solutions And Solubility Powerpoin vrogue.co Do Solvents Dissolve Solutes Solvents that are nonpolar will dissolve nonpolar solutes. When one substance dissolves into another, a solution is formed. The substance that dissolves is called. Solvation is the process in which molecules of a solvent attract the particles of a solute. A solution forms when one substance is dissolved by another. Solute the solid (or occasionally a gas) which dissolves into. Do Solvents Dissolve Solutes.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Do Solvents Dissolve Solutes When one substance dissolves into another, a solution is formed. A solution forms when one substance is dissolved by another. The solute is the substance that is being dissolved, while the. The substance that dissolves is called. Solvents that are very polar will dissolve solutes that are very polar or even ionic. A solution is a homogenous mixture consisting of. Do Solvents Dissolve Solutes.
From pressbooks.online.ucf.edu
12.1 The Dissolution Process Chemistry Fundamentals Do Solvents Dissolve Solutes Solvents that are very polar will dissolve solutes that are very polar or even ionic. When one substance dissolves into another, a solution is formed. Solvents that are nonpolar will dissolve nonpolar solutes. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. A solution is a homogenous mixture consisting of a solute dissolved into a. Do Solvents Dissolve Solutes.
From www.liveworksheets.com
Solute solvent solution worksheet Live Worksheets Do Solvents Dissolve Solutes Solvents that are nonpolar will dissolve nonpolar solutes. Solvents that are very polar will dissolve solutes that are very polar or even ionic. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. When one substance dissolves into another, a solution is formed. Solvation is the process in which molecules of. Do Solvents Dissolve Solutes.
From www.sciencephoto.com
Example of a solute dissolving in solvent Stock Image A350/0109 Do Solvents Dissolve Solutes The solute is the substance that is being dissolved, while the. The solute is the substance that is being dissolved, while the. The substance that dissolves is called. A solution is a homogenous mixture consisting of a solute dissolved into a solvent. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. Solvents that are nonpolar. Do Solvents Dissolve Solutes.
From www.youtube.com
How does a Solute Dissolve in a Solvent? Solutions Chemistry Don Do Solvents Dissolve Solutes The solute is the substance that is being dissolved, while the. The substance that dissolves is called. Solvents that are nonpolar will dissolve nonpolar solutes. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. A solution forms when one substance is dissolved by another. A solution is a homogenous mixture consisting of a solute dissolved. Do Solvents Dissolve Solutes.